Abstract
Introduction: Light chain (AL) amyloidosis is a plasma cell disease characterized by the deposition of insoluble amyloid fibrils into organs leading to organ dysfunction and death. The analysis at 6 and 12 months of the ANDROMEDA study (NCT03201965) showed that the addition of subcutaneous (SC) daratumumab to the standard of care combination of bortezomib, cyclophosphamide, and dexamethasone (VCd) was superior to VCd alone, with higher rates of hematologic complete response (CR) and an acceptable safety profile. Based on these findings daratumumab with VCd (D-VCd) was approved for newly diagnosed AL amyloidosis in January 2021 in the US and June 2021 in the EU. Here, we present data from the analysis at 18 months of the ANDROMEDA study.
Methods: ANDROMEDA is a randomized, open-label, active-controlled phase 3 study including patients with newly diagnosed AL amyloidosis with measurable hematologic disease, ≥1 involved organ, cardiac stage I-IIIA, estimated glomerular filtration rate ≥20 mL/min, and absence of symptomatic multiple myeloma. Patients were randomized (1:1) to D-VCd or VCd for 6 28-day cycles. Bortezomib (1.3 mg/m 2), cyclophosphamide (300 mg/m 2 up to 500 mg per week), and dexamethasone (20-40 mg) were administered weekly. SC daratumumab (1800 mg co-formulated with recombinant human hyaluronidase PH20 in 15 mL) was administered once weekly in cycles 1 and 2 and every 2 weeks in cycles 3 to 6. Patients in the D-VCd arm received only SC daratumumab after cycle 6, every 4 weeks (up to a total of 24 cycles from first dose). The primary endpoint was overall (ie, at any time) hematologic CR rate, defined here as normalization of free light chain (FLC) levels and ratio (FLCr) and negative serum and urine immunofixation, confirmed at a subsequent visit; normalization of uninvolved FLC level and FLCr were not required if involved FLC was lower than the upper limit of normal. Secondary endpoints included major organ deterioration progression-free survival (PFS), organ response rate, time to hematologic response, overall survival (OS), and safety.
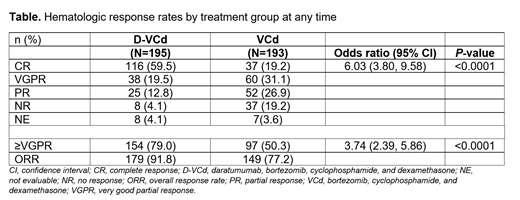
Results: A total of 388 patients were randomized to receive D-VCd (N=195) or VCd alone (N=193). At the May 2021 clinical cutoff, the median duration of treatment for D-VCd and VCd arms was 21.3 and 5.3 months, respectively. In the D-VCd arm, 149 patients (77.2%) received daratumumab monotherapy after completing 6 cycles of D-VCd; of those, 17 (11.4%) were still receiving treatment. The rates of deep hematological responses favored the D-VCd treatment arm (Table). At a median follow-up of 25.8 months, the rate of hematologic CR was significantly higher in the D-VCd arm vs the VCd arm (59.5% vs 19.2%; odds ratio [95% confidence interval (CI)], 6.03 [3.80-9.58]; P<0.0001). Similarly, more patients achieved a very good partial response or better (≥VGPR) (D-VCd vs VCd, 79.0% vs 50.3%; odds ratio [95% CI], 3.74 [2.39-5.86]; P<0.0001). Among patients who responded, the median time from randomization to ≥VGPR was shorter in the D-VCd arm (0.56 months) vs the VCd arm (0.82 months). Comparable to the cardiac response analysis at 6 months (D-VCd vs VCd, 42% vs 22%), greater cardiac response rates were achieved with D-VCd compared with VCd at 18 months (53% vs 24%). Similarly, renal response rates remained superior with D-VCd vs VCd alone at 18 months (58% vs 26% compared with 6 months [54% vs 27%]). A total of 79 deaths occurred (D-VCd, N=34; VCd, N=45). In the D-VCd arm, only 1 additional grade 3/4 treatment-emergent adverse event occurred over 18 months vs 12 months (119 [61.7%] vs 118 [61.1%] patients) and no additional infusion-related reactions were reported. OS will be analyzed and major organ deterioration PFS will be updated after approximately 200 events have occurred.
Conclusions: These results demonstrate the sustained clinical benefits of D-VCd vs VCd in terms of hematologic and organ responses with longer follow-up, although it should be noted that many patients in the D-VCd arm received daratumumab monotherapy following 6 cycles of D-VCd, while patients in the VCd group stopped study treatment. Nevertheless, the study continues to support the use of D-VCd over VCd alone in patients with newly diagnosed AL amyloidosis. Following its recent approval, D-VCd represents a new SOC for patients with AL amyloidosis.
Comenzo: Prothena Biosciences: Consultancy, Research Funding; Takeda: Research Funding; Unum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Research Funding; Caelum: Consultancy, Research Funding; Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees; Janssen: Patents & Royalties: WO2016187546A1, Research Funding. Palladini: Pfizer: Honoraria; Siemens: Honoraria; Janssen Global Services: Honoraria, Other: advisory board fees. Kastritis: Pfizer: Consultancy, Honoraria, Research Funding; Takeda: Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Genesis Pharma: Honoraria. Minnema: Janssen: Consultancy; BMS: Consultancy; Kite/Gilead: Consultancy; Celgene: Other: Travel expenses; Alnylam: Consultancy; Cilag: Consultancy. Wechalekar: Amgen: Research Funding; Janssen: Consultancy; Takeda: Honoraria; Celgene: Honoraria; Caelum Biosciences: Other: Clinical Trial Funding; Alexion, AstraZeneca Rare Disease: Consultancy. Jaccard: Pfizer: Honoraria; Abbvie: Honoraria; Janssen: Membership on an entity's Board of Directors or advisory committees. Dispenzieri: Janssen: Consultancy, Research Funding; Sorrento Therapeutics: Consultancy; Oncopeptides: Consultancy; Alnylam: Research Funding; Pfizer: Research Funding; Takeda: Research Funding. Lee: Karyopharm: Consultancy; Celgene: Consultancy; Takeda Pharmaceuticals: Consultancy, Research Funding; Amgen: Consultancy, Research Funding; GlaxoSmithKline: Consultancy, Research Funding; Sanofi: Consultancy; Genentech: Consultancy; Legend Biotech: Consultancy; Janssen: Consultancy, Research Funding; Oncopetides: Consultancy; Bristol Myers Squibb: Consultancy; Regeneron: Research Funding. Sanchorawala: Oncopeptide: Research Funding; Karyopharm: Research Funding; Sorrento: Research Funding; Pfizer: Honoraria; Proclara: Membership on an entity's Board of Directors or advisory committees; Regeneron: Membership on an entity's Board of Directors or advisory committees; Caelum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Prothena: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; Celgene: Research Funding. Gibbs: AbbVie: Consultancy; Janssen, Celgene, Amgen, Takeda, BMS and Pfizer: Consultancy, Honoraria. Mollee: Janssen, Pfizer: Research Funding; Amgen, BMS, Janssen, Caelum, EUSA, Pfizer, SkylineDx, Takeda: Membership on an entity's Board of Directors or advisory committees, Other: No personal fees received. Venner: BMS: Honoraria; Amgen: Research Funding; Celgene: Research Funding; Amgen: Honoraria; Takeda: Honoraria; Janssen: Honoraria; Sanofi: Honoraria; Pfizer: Honoraria. Schönland: Janssen: Honoraria, Other: Travel grants, Research Funding; Pfizer: Honoraria; Takeda: Honoraria, Other: Travel grants; Prothena: Honoraria, Other: Travel grants; Sanofi: Research Funding. Suzuki: Abie: Honoraria; Sanofi: Honoraria; Novartis: Honoraria; ONO: Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Janssen: Consultancy, Honoraria. Kim: BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Cibeira: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Akcea: Honoraria, Membership on an entity's Board of Directors or advisory committees. Beksac: Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Libby: Genentech: Research Funding; BMS: Research Funding; GSK: Research Funding; Janssen: Consultancy, Research Funding. Valent: Caelum Biosciences: Other: Clinical Trial Funding; Celgene Corporation: Speakers Bureau; Takeda Pharmaceuticals: Speakers Bureau; Amgen: Speakers Bureau. Hungria: Amgen, BMS, Celgene, Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings/travel ; Abbvie: Honoraria; Sanofi: Honoraria, Other: Support for attending meetings/travel ; Takeda: Honoraria. Wong: Amgen: Consultancy; Genentech: Research Funding; Fortis: Research Funding; Janssen: Research Funding; GloxoSmithKlein: Research Funding; Dren Biosciences: Consultancy; Caelum: Research Funding; BMS: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees. Rosenzweig: Janssen: Consultancy, Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Akcea: Speakers Bureau; Takeda: Speakers Bureau; Onocopeptides: Speakers Bureau. Bumma: Amgen, Sanofi: Speakers Bureau; Janssen, Oncopeptides, Sanofi: Consultancy. Tran: Janssen: Current Employment, Current equity holder in publicly-traded company. Qin: Janssen: Current Employment. Khaled: Jazz: Honoraria; Astellas: Honoraria; Alexion: Honoraria, Speakers Bureau; Omeros: Honoraria; Janssen: Current Employment. Vermeulen: Janssen: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal